HTML
Research Article - (2021) Volume 11, Issue 8
Formulation and Evaluation of Gastroretentive Mucoadhesive Tablet of Gliclazide by using Synthetic Polymers
Singh Vikas* and Pallavi Joshi*Correspondence: Singh Vikas, Department of Pharmaceutical Sciences, Shri Guru Ram Rai University, Uttarakhand, India, Email:
Received: 04-Oct-2021 Published: 25-Oct-2021
Abstract
Gliclazide is an oral anti hyperglycemic agent used to treat non-insulin dependent diabetes mellitus. Aim of the present work is to formulate and evaluate mucoadhesive tablet of Gliclazide by using synthetic polymers like HPMC K4M, and carbopol 940. The tablets were formulated by direct compression method and then evaluated pre-compression and post compression such as hardness, friability, thickness, weight variation, drug content, drug release, FTIR, mucoadhesive strength. Polymers and drug have no interactions in IR. The formulation was found to have good pre-formulation characteristics. Formulation (F3) showed good mucoadhesive strength (22.720 g) and maximum drug release of 57.4% in 10 hours. The drug content has shown 96.56%. All the evaluation parameters given the positive result and comply with the standards. Advantage of mucoadhesive tablet is increase the residence time and control the release of drug as well as enhance bioavailability. It also improves patient compliance, drug efficacy, and therapeutic effects.
Keywords
Gliclazide, Mucoadhesive tablet, In vitro Drug release, Synthetic polymers
Introduction
The primary goal of sustain drug delivery system is to deliver drug for longer period of time to achieve better bioavailability, which should be predictable and reproducible. But it is difficult due to the number of physiological problems such as fluctuation in the gastric emptying process, narrow absorption window and stability problem. To overcome these problems, different approaches have been proposed to retain dosage form in stomach. These include mucoadhesive systems, swelling and expanding systems, floating system, and other delayed gastric emptying devices [1, 2].
Gastric mucosa is the preferred site for both system local and systemic action. The mucosa has a rich blood supply and relatively permeable. In the mucoadhesive drug delivery systems mucoadhesion is the key element. In this adhesion ability of a biological or synthetic material to stick to a mucus membrane, resulting in adhesion of the material to the tissue for a protracted period of time. Various binding agents can be useful in achieving various tablet mechanical strength and drug release [3].
Gliclazide bind to the β cell Sulfonyl Urea Receptor (SUR1). This binding blocks the ATP sensitive potassium efflux leads to depolarization of the β cell. This opens voltage-dependent calcium channels in the β cell resulting in calmodium activation, which in turn leads to exocytosis of insulin [4].
Materials and Methods
List of materials used (Table 1).
| S. No. | Name | Supplier/Manufacturer |
|---|---|---|
| 1 | Gliclazide | Gift Sample from Pharmaceuticals |
| 2 | Methanol | Central Dug House Pvt. Ltd |
| 3 | HPMC K4M | Central Dug House Pvt. Ltd |
| 4 | Carbopol 940 | Central Dug House Pvt. Ltd |
| 5 | MCC | Central Dug House Pvt. Ltd |
| 6 | Lactose | Central Dug House Pvt. Ltd |
| 7 | Talc | Central Dug House Pvt. Ltd |
| 8 | Magnesium Stearate | Central Dug House Pvt. Ltd |
| 9 | Hydrochloride | Central Dug House Pvt. Ltd |
Formulation of mucoadhesive tablets
In the case of prepared tablet, the method of direct pressing is used. Gliclazide was mixed together with different ingredients according to formula. The powder mixture was then lubricated with magnesium stearate and compressed with a tablet punching machine. Micro crystalline cellulose and lactose was used to keep the tablet weight constant and optimize poor solubility of the drug (Table 2).
| Batches | HPMC K4M | Carbopol 940 | Micro C.C. | Lactose | Talc | Magnesium Stearate | Drug | Total |
|---|---|---|---|---|---|---|---|---|
| F1 | 60 | 45 | 58 | 37 | 10 | 10 | 80 | 300 |
| F2 | 45 | 30 | 40 | 85 | 10 | 10 | 80 | 300 |
| F3 | 75 | 30 | 40 | 55 | 10 | 10 | 80 | 300 |
| F4 | 45 | 60 | 40 | 55 | 10 | 10 | 80 | 300 |
| F5 | 75 | 60 | 40 | 25 | 10 | 10 | 80 | 300 |
| F6 | 45 | 60 | 76 | 19 | 10 | 10 | 80 | 300 |
| F7 | 35 | 45 | 58 | 62 | 10 | 10 | 80 | 300 |
| F8 | 85 | 45 | 58 | 12 | 10 | 10 | 80 | 300 |
| F9 | 60 | 70 | 58 | 12 | 10 | 10 | 80 | 300 |
Evaluation tests
Pre-compressional parameters:
• Bulk density
• Tapped density
• Angle of repose
• Hasusner’s ratio
• Carr’s consolidation index
Bulk density: It is the ratio of total mass of powder to the bulk volume of powder. Accurately weighed batch (F1–F9) powder was placed in 10 mL graduated measuring cylinder. Initial volume was observed. The Db and was calculated in gm/mL using following formulae,
Db=M/Vb
Where, Db=Bulk density, M=Mass of the powder, Vb=Bulk volume of powder
Tapped density: Accurately weighed batch (F1-F10) powder was placed in 10 mL graduated measuring cylinder. The cylinder was tapped initially 100 times from a distance of 14+2 mm. The tapped volume was measured to the nearest graduated unit. Again the tap volume was measured to the nearest graduated unit. The Dt were calculated in g/mL using following formulae,
Dt=M/Vt
Where, Dt=Tapped density, Vt=Tapped volume of the powder, Dt=Tapped density, M=Mass of the powder
Angle of repose: Good flow properties are critical for the development of any pharmaceutical tablets, capsule or powder formulations. Angle of repose is defined as the maximum angle possible between the surface of the pie of powder and horizontal plane. It is performed to determine the flow property of powder done by the funnel method. The powder mass was allowed to flow through the funnel orifice, kept vertically to a plane paper kept on horizontal surface, giving a heap angle of powder on a paper. The diameter of the powder cone was measured and angle of repose was calculated using the following equation
Tan θ=h/r
Where, h and r are the height and radius of the powder cone, respectively.
Hasusner’s ratio: Hasusner’s ratio carried out by tapped density divided bulk density.
Hasusner´s ratio=Tapped density/Bulk density
Carr’s consolidation index: Carr developed an indirect method of measuring powder flow from bulk densities. The % compressibility of the powder was direct measure of the potential powder arch or bridge strength and stability. Carr’s index of each formulation was calculated using the given formula.
Carr’s index (%)=[(Dt –Db) × 100]/Dt
Post compression parameters
Appearance: The tablets were checked for presence of cracks, pinholes etc. There should be uniformity in the color and the dimensions of the tablets.
Hardness: This test is used to check the hardness of the tablet, which may undergo chipping or breakage during storage, transportation, and handling. In this, three tablets were selected randomly and the hardness of each tablet was measured with Monsanto hardness tester. The hardness is usually measured in terms of kg/cm2.
Thickness: Thickness of tablet was important for uniformity of the tablet size. In this three tablets were selected randomly and the hardness of each tablet was measured with using screw gauze.
Friability test: Friability test was carried out to evaluate the hardness and stability instantly. In roche friabilator, 10 tablets were weighed (W0) initially and put in a tumbling and rotating apparatus drum. Then they were subjected for completion of 4 min or 100 rpm, the tablets were again weighed. The % loss in weight or Friability (F) was calculated by the formula given below.
% Weight Loss=W1-W2/W1 × 100
Where, W1=Initial weight, W2=Final weight
Weight variation: This test was performed to maintain the uniformity of weight of each tablet, which should be in the prescribed range. This was done by weighing 10 tablets at random and average weight was calculated. Not more than two of individual weight deviates from the average weight. The weight data from the tablets were analyzed for sample mean and percent deviation [5].
Weight variation=(IW-AW)/AW × 100
Where, IW=Individual weight, AW=Average weight
Uniformity of drug content: The content uniformity was mandatory for tablets. This test was performed by taking five tablets were selected randomly, weighed and powdered. A tablet triturate equivalent to 40 mg of drug weighed accurately, dissolved in 10 mL methanol then final volume made up to 100 mL by using pH 1.2 buffers [6]. Further dilutions were done suitably and absorbance was measured at 230 nm using UV spectrophotometer.
Swelling index: The swelling of tablet involves the absorption of a liquid resulting in an increase in weight and volume. Liquid uptake by the particle results to saturation of capillary spaces within the particles. The liquid enters the particles through pores and bind to large molecule breaking the hydrogen bond and resolution in the swelling of particle. One tablet from each batch was weighed and placed in a Petri plate containing 25 mL of pH 1.2 buffer solutions [7]. After each 2 hours interval the tablet was removed from plate, removes excess of buffer by using filter paper and weighed again up to 24 hours. The swelling index was calculated using following formula.
In vitro dissolution studies: Dissolution tests were performed in USP dissolution eight dissolution apparatus II (paddles) at 37 ± 0.5°C. The baskets were rotated at a speed of 50 rpm. The test was performed in 37 ± 0.5°C with a rotation speed of 50 rpm using 900 mL of 0.1 N HCl, pH 1.2, as a dissolution medium. According to the sampling plan, samples of 5 mL were withdrawn till 24 hours and immediately replaced with an equal volume of the respective dissolution medium maintained at 37 ± 0.5°C. Test samples were filtered through Whatman filter paper for Gliclazide at 230 nm using a blank solution as reference with a UV-VIS double-beam spectrophotometer.
In vitro mucoadhesive strength: Mucoadhesion strength of the tablet was measured by using sheep stomach mucosa as model mucosal membrane. Fresh sheep stomach mucosa was obtained from a local slaughter house and was used within 2-3 hours of slaughtering. The mucosal membrane was washed with distilled water and then with pH 1.2.
The mucoadhesive strength measurement apparatus was fabricated locally. The mucoadhesive strength of the tablets was determined using this locally fabricated apparatus [8]. The weight at which the tablet was detached was recorded. The mean value of three trials was taken for each set of formulations. After each measurement, the tissue was gently and thoroughly washed with phosphate buffer and left for 5 minutes before placing a new tablet to get appropriate results for the formulation.
Results
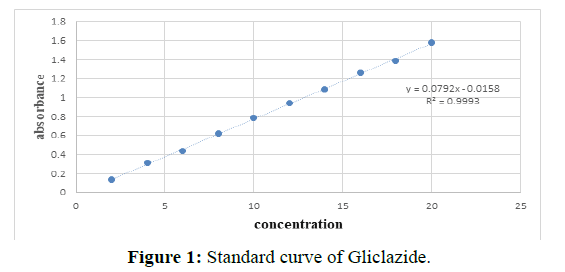
Preparation of calibration curve in 0.1N HCL (Tables 3 and 4 and Figure 1).
| S. No. | Concentration (µg/ml) | Absorbance (nm) |
|---|---|---|
| 1 | 2 | 0.1428 |
| 2 | 4 | 0.312 |
| 3 | 6 | 0.436 |
| 4 | 8 | 0.6191 |
| 5 | 10 | 0.7845 |
| 6 | 12 | 0.945 |
| 7 | 14 | 1.0844 |
| 8 | 16 | 1.2581 |
| 9 | 18 | 1.3881 |
| 10 | 20 | 1.5784 |
| S.No. | Parameters | Observation |
|---|---|---|
| 1 | Absorbance maxima | 230 nm |
| 2 | Slope | 0.079 |
| 3 | Intercept | 0.015 |
| 4 | Coefficient of correlation | 0.9992 |
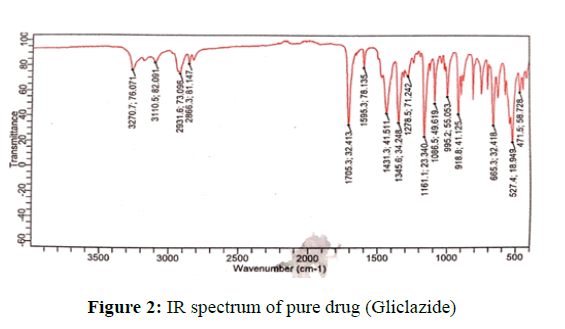
Infrared spectrum analysis: In the IR study, it was found that there was no interaction exhibited between Gliclazide and polymers used (Figures 2 and 3) and (Tables 5-8).
| Formulation code | Bulk density (g/ml) | Tapped density(g/ml) | Hausner’s ratio | Compressibility index (%) | Angle of repose |
|---|---|---|---|---|---|
| F1 | 0.4176 | 0.5067 | 1.1 | 14.23 | 23 0.11 |
| F2 | 0.4108 | 0.5243 | 1.2 | 13.43 | 24.58 |
| F3 | 0.4201 | 0.5389 | 1.1 | 15.51 | 21.43 |
| F4 | 0.4283 | 0.5361 | 1.1 | 12.5 | 22.43 |
| F5 | 0.4387 | 0.512 | 1.1 | 13.72 | 23.52 |
| F6 | 0.4261 | 0.4849 | 1.2 | 13.89 | 22.12 |
| F7 | 0.435 | 0.5186 | 1.1 | 10.49 | 20.38 |
| F8 | 0.4283 | 0.4976 | 1.1 | 11.12 | 21.76 |
| F9 | 0.4289 | 0.4865 | 1.1 | 10.85 | 20.93 |
| Formulation Code | Weight Variation (mg) | Drug Content (%) | Friability (%) | Hardness (Kg/Cm2) | Thickness (mm) |
|---|---|---|---|---|---|
| F1 | 299 | 95.03 | 0.73 | 5.32 | 2.312 |
| F2 | 298 | 95.21 | 0.88 | 5.12 | 2.462 |
| F3 | 301 | 94.56 | 0.91 | 6.49 | 2.214 |
| F4 | 299 | 96.37 | 0.89 | 5.5 | 2.215 |
| F5 | 296 | 94.2 | 0.82 | 5.43 | 2.22 |
| F6 | 302 | 94.6 | 0.72 | 6.21 | 2.267 |
| F7 | 299 | 93.32 | 0.82 | 5.56 | 2.712 |
| F8 | 299 | 90.3 | 0.9 | 5.17 | 2.671 |
| F9 | 297 | 90.24 | 0.78 | 5.84 | 2.54 |
| Formulation code | Mucoadhesive strength (g) | Mucoadhesion force (N) |
|---|---|---|
| F1 | 23.471 | 2.302036 |
| F2 | 22.3 | 2.187184 |
| F3 | 22.72 | 2.228378 |
| F4 | 21.35 | 2.094008 |
| F5 | 20.58 | 2.018486 |
| F6 | 23.89 | 2.343131 |
| F7 | 22.576 | 2.214254 |
| F8 | 22.68 | 2.224454 |
| F9 | 24.053 | 2.359118 |
| Form. code | % Swelling index Time (hours) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 hrs |
1 hrs | 2 hrs | 3 hrs | 4 hrs | 5 hrs | 6 hrs | 7 hrs | 8 hrs | |
| F1 | 0 | 5 | 8.42 | 12.65 | 15.9 | 18.29 | 22.65 | 29 | 31.43 |
| F2 | 0 | 4 | 8.67 | 11.28 | 13.7 | 17.76 | 21.98 | 28 | 31.67 |
| F3 | 0 | 4 | 5.76 | 10.37 | 14.1 | 18.87 | 23.76 | 27 | 33.76 |
| F4 | 0 | 4 | 8.83 | 12.43 | 13.8 | 20.34 | 24.48 | 30 | 33.99 |
| F5 | 0 | 4 | 7.76 | 11.22 | 17.6 | 20.1 | 24.31 | 29 | 32.12 |
| F6 | 0 | 5 | 8.75 | 10.98 | 15.7 | 18.65 | 24.11 | 28 | 32.87 |
| F7 | 0 | 4 | 7.65 | 11.67 | 13.1 | 18.06 | 23.9 | 29 | 35.78 |
| F8 | 0 | 5 | 8.98 | 12.39 | 17.2 | 19.91 | 22.78 | 28 | 35.1 |
| F9 | 0 | 4 | 7.21 | 11.25 | 16.5 | 20.27 | 25.01 | 29 | 35.89 |
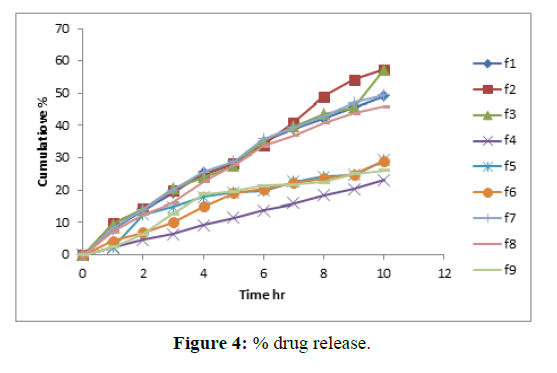
In vitro dissolution studies: In vitro drug release studies were performed by using USP XXIII dissolution test apparatus-II at 50 rpm using 900 mL of 1.2 pH buffer maintained at 37 ± 0.5°C as the dissolution medium (Tables 9 and 10 and Figure 4).
| Time (Hrs) | Dissolution media 0.1N HCL (% drug release) Formulation Code | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| 1 | 8% | 9.70% | 9.50% | 2.25% | 2.32% | 4.33% | 8.30% | 7.00% | 2.30% |
| 2 | 13.90% | 14.20% | 14.00% | 4.67% | 12.50% | 6.95% | 14.00% | 12.50% | 6.30% |
| 3 | 19.10% | 19.80% | 20.50% | 6.46% | 14.70% | 10.08% | 20.00% | 16.10% | 12.70% |
| 4 | 25.40% | 24.40% | 24.00% | 9.09% | 17.90% | 14.87% | 25.60% | 22.70% | 18.80% |
| 5 | 27.60% | 28.40% | 27.50% | 11.40% | 19.30% | 19.20% | 28.90% | 27.90% | 19.60% |
| 6 | 35.10% | 34.10% | 35.40% | 13.80% | 19.80% | 20.40% | 35.80% | 33.70% | 21.40% |
| 7 | 39.10% | 41.30% | 39.70% | 15.90% | 22.70% | 22.20% | 39.30% | 36.90% | 21.80% |
| 8 | 42.20% | 48.90% | 43.50% | 18.40% | 24.20% | 23.60% | 42.80% | 40.80% | 22.40% |
| 9 | 45.70% | 54.20% | 45.70% | 20.30% | 24.70% | 24.80% | 47.30% | 43.70% | 25.00% |
| 10 | 49.20% | 57.40% | 57.40% | 23.20% | 29.20% | 29.10% | 49.50% | 45.90% | 26.10% |
| FORMLN. CODE | Mathematical models (kinetics) | ||||
|---|---|---|---|---|---|
| Zero order ® | First order ® | Higuchi (R) | Korsmeyer-Peppas | ||
| (N) | (R) | ||||
| F1 | 0.9848 | 0.997 | 0.9 | 0.79 | 0.9973 |
| F2 | 0.9935 | 0.9786 | 0.9 | 0.8 | 0.9069 |
| F3 | 0.986 | 0.9691 | 0.9 | 0.76 | 0.9873 |
| F4 | 0.9994 | 0.9973 | 0.9 | 1.007 | 0.9991 |
| F5 | 0.9049 | 0.9274 | 0.9 | 0.906 | 0.8383 |
| F6 | 0.968 | 0.9775 | 0.9 | 1.8758 | 0.9941 |
| F7 | 0.984 | 0.9976 | 0.9 | 0.7911 | 0.9981 |
| F8 | 0.984 | 0.9955 | 0.9 | 0.845 | 0.9949 |
| F9 | 0.895 | 0.9121 | 0.9 | 1.0109 | 0.9138 |
Discussion
Formulation and evaluation parameters have been performed in satisfactory data. The aim of study to prolong residence time and bioavailability. The percent drug content of the optimized formulation was found to be 90.24 w/w to 96.37 w/w. Hardness of the tablets was found to be in a range of 5.12 to 6.49 kg/cm2 and it was found that hardness will increase with increase the amount of carbopol 940. Weight variation of the tablets range is found to be 296.6 to 301.7 and % deviation in a specified limit. Hence all formulations complied with the test for weight uniformity. The entire tablet was circular with no cracks and with average thickness of 2.214 mm to 2.712. Friability value also considered and weight loss was found to less than 1%. All the formulations were studied and its results indicate that all the formulations possess good swelling index. The mucoadhesion force was affected by amount of polymer. The highest mucoadhesive force by optimum formulation was found to be 2.09 N to 2.35.
Conclusion
The present study was aimed to develop prolong release stable, pharmaceutically equivalent formulation of gliclazide using polymers HPMC K4M, and Carbopol 940 optimized by 32 full factorial design.
The satisfactory formulation shows a zero order drug release profile depending on the regression value and shows a satisfactory dissolution profile. Slow, controlled release of Gliclazide over a period of 10 hrs was obtained from F3 formulation.
Further work is to be carried out in order to determine its efficacy and safety by long term pharmacokinetic and pharmacodynamics studies.
Conclusion
The present study was aimed to develop prolong release stable, pharmaceutically equivalent formulation of gliclazide using polymers HPMC K4M, and Carbopol 940 optimized by 32 full factorial design.
The satisfactory formulation shows a zero order drug release profile depending on the regression value and shows a satisfactory dissolution profile. Slow, controlled release of Gliclazide over a period of 10 hrs was obtained from F3 formulation.
Further work is to be carried out in order to determine its efficacy and safety by long term pharmacokinetic and pharmacodynamics studies.
References
- Gangadharappa HV, Pramod kumar TM, Shiva Kumar HG. Ind. J. Pharm. Edu. Res. 2007; 41(4):295-303.
- Santus G, Lazzarini G, Bottom G. Eur. J. Pharm. Biopharm. 1997; 44(9):39-52.
- Singh J, Pawan D. Int. J. Pharm. Sci. Res. 2013; 4(3):916-927.
- Jasti B, Li X, Cleary G. Pharmatech. 2003; 19(1):194-197.
- Lachman L, Liberman HA. Varg. Pub. House. 1991; 57:297-303.
- Pandit V, Sarasija S, Joshi H. Int. J. Pharm. Bio. Sci. 2010; 1(2):13-23.
- Ingle US, Bankar VH, Gaikwad PD, Pawar SP. Int. J. Bio. Pharm. Tech. 2011; 2 (2):301-06.
- Senthil V, Kumar R, Lavanya K, Rathi D, Venkatesh N, Ganesh GN, Jawahar K. J. Pharm. Res. 2010; 3(8):1961-1966.
Manuscript Submission
Submit your manuscript at Online Submission System
Google scholar citation report
Citations : 1101
International Journal of Pharmacy received 1101 citations as per google scholar report
International Journal of Pharmacy peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- HINARI
- Index Copernicus
- Google Scholar
- The Global Impact Factor (GIF)
- Polish Scholarly Bibliography (PBN)
- Cosmos IF
- Open Academic Journals Index (OAJI)
- Directory of Research Journal Indexing (DRJI)
- EBSCO A-Z
- OCLC- WorldCat
- MIAR
- International committee of medical journals editors (ICMJE)
- Scientific Indexing Services (SIS)
- Scientific Journal Impact Factor (SJIF)
- Euro Pub
- Eurasian Scientific Journal Index
- Root indexing
- International Institute of Organized Research
- InfoBase Index
- International Innovative Journal Impact Factor
- J-Gate