HTML
Research Article - (2021) Volume 11, Issue 10
Evaluation of Anti-oxidant and Wound Healing Property of Anogeissus acuminata Leaves
Mohd Amer AK1, Iqbal MM2*, Rehman MAU2, Asadulla SB3 and Jat RK1*Correspondence: Iqbal MM, Department of Pharmacy Practice, MESCO College of Pharmacy, Hyderabad, India, Email:
Received: 02-Nov-2021 Published: 23-Nov-2021
Abstract
Plants are the “sleeping giant of drugs development”. They form a reservoir of potential useful untapped sources of drugs that have been serving mankind since dawn of civilization. Hence it is necessary to explore the possibility of using the traditional medicines with proper chemical and pharmacological profiles.
Wound healing is a complex and dynamic process involving the phases of haemostasis, inflammation, granulation and maturation. Thermal injury is one of the most severe forms of trauma that affects the organism both locally and systematically. Localized burns affect the skin and hermits ability to act both as a protective barrier to the environment and as an immune organ. Burn wound healing is a complex process consisting of an early phase of abrupt energy depletion and necrosis, followed by inflammatory phase, delayed cell death, formation of granulation tissue, matrix formation and remodelling.
In the present study the systemic evaluation procedure is followed for determining any possible excision, burn wound healing and antioxidant activities of Anogeissus acuminate leaves were carried out. Alcoholic and aqueous extracts of Anogeissus acuminate leaves possessed maximum antimicrobial activity. The LD50 of both the extracts were found to be 3000 mg/kg b.w (p.o.).
The wound healing studies of alcoholic and aqueous extracts showed significant wound healing activity and anti-oxidant property at 300 mg/ kg dose as compared to control. From the dead space wound studies, it was found that alcoholic and aqueous extracts of Anogeissus acuminata significantly increased the tensile strength of granuloma and there was significant increase in the weight of cotton pellet granuloma than the control. Wound healing activity and histopathological studies of the leaves of Anogeissus acuminata on excision wound model, incision wound model, formation of granuloma, collagen contents in the tissues were significant when compared to control.
Keywords
Anti-oxidant, Histopathological studies, Anogeissus acuminate, Traditional medicines
Introduction
The relationship between man and plants has been close throughout the development of human culture with the disease in the understanding of human disease there has been continued interest in the drugs from the plant kingdom [1].
The demand for herbal products is growing exponentially throughout the world and major pharmaceutical companies are currently conducting extensive research on plant material for their potential medicinal values [2].
An antioxidant is a molecule capable of slowing or preventing the oxidation of other molecules. Although oxidation reactions are crucial for life, they can also be damaging; hence, plants and animals maintain complex systems of multiple types of antioxidants, such as glutathione, Vitamin C and Vitamin E as well as enzymes such as catalase, superoxide dismutase and various peroxidases. Low levels of antioxidants, or inhibition of the antioxidant enzymes, cause oxidative stress and may damage or kill the cells.
The term antioxidant originally was used to refer specifically to a chemical that prevented the consumption of oxygen. The possible mechanisms of action of antioxidants were first explored when it was recognized that a substance with anti-oxidative activity is likely to be one that is itself readily oxidized. Research into how Vitamin E prevents the process of lipid peroxidation led to the identification of antioxidants as reducing agents that prevent oxidative reactions, often by scavenging reactive oxygen species before they can damage cells [3].
Wounds may be defined as loss or breaking of cellular and anatomic or functional continuity of living tissues [4]. Wounds are inescapable event in life. Wounds may arise due to physical, chemical or microbial agents. Wound healing is the restoration of integrity of injured tissues. It is a dynamic process, involving sequence of events which takes place in an orderly way, i.e. inflammatory repair, closure, remodelling and final healing. Wound healing refers to replacement of dead tissue by visible tissue [5].
Granuloma is a vital step in wound healing. The rate of granulation can be directly correlated to the wound healing. Hence measurement of extent of granuloma deposition in dead space wounds serves as a meaningful parameter. These studies can be extended for histological studies and wound tensile strength measurement [6,7].
Anogeissus acuminata belongs to Combretaceae family and is a wild evergreen tree of variable size, found both wild and cultivated throughout India. Anogeissus acuminata is known to be a rich source of carbohydrates, fat, protein, amino acids, anthocyanin’s, leucoanthocyanins, saponins and alkaloids.
The ground seeds on extraction with petroleum ether gave 33 percent of bright yellowish oil which contains oleic and linoleic. The seed also contains myristic, palmitic acid, β-sitosterol, stigmasterol, jujubosides A and B (saponins), spinosin and its derivatives.
Reports suggest that flavonoid containing herbal drugs had an effect on collagen fibres pre-treated with acrylic polymer followed by treatment of tannin containing herb exhibited an increase in hydrothermal stability, which reveals that tannin containing herbs stabilizes type- 1 collagen which is unique connective tissue protein which promote burn wound healing. Flavonoids and tannin containing compounds also help in rapid cleaning of purulent wounds and shortening of duration of treatment.
Materials and Methods
Acute toxicity study
Acute oral toxicity:
• Acute toxic class method: The acute oral toxicity study was carried out as per the guidelines set by Organization for Economic Cooperation and Development (OECD), received draft guidelines 423, received from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), ministry of social justice and empowerment, Government of India [8].
• The method enables a judgment with respect to classifying the test substances to one of the series of toxicity classes defined by fixed LD50 cut off values. The LD 50 values for both extracts were 3000 mg/kg b.w., so the dose had been selected to be 300 mg/kg. At this dose no toxicity was found. Absence or presence of compound related mortality of the animals dosed at one step will determine the next step [9].
• Selection of animal species: Healthy young albino westar rats of either sex weighing between 100-200 gm were used for acute toxicity study to determine LD50 of various extracts. Totally they were four groups, each groups consists of three animals.
• Housing and feeding condition: The temperature in the experimental room was around 250°C. Lightning was artificial, the sequence being 12 hours dark, 12 hour light. The conventional laboratory diet was fed, with an unlimited supply of drinking water.
• Preparation of animals and doses: The animals were randomly selected, marked to permit individual identification, and kept in their cages for seven days prior to dosing to allow for acclimation to the laboratory condition. All the extracts were prepared as a suspension by triturating with 2% of gum acacia.
• Administration of doses: The test substances are administered in a single dose by gavage using a stomach tube. Animals were fasted prior to dosing, following period fasting, the animals were weight and test substance was administered. After the dose was administered, food was withheld for a further 3-4 hours in rats.
• Number of animals and dose levels: In each steps three animals were used in each group. Since there was no information on the substance to be tested (i.e. extracts), starting dose was 40 mg/ kg body weight up to 3000 mg/kg body weight. 1/10th of this lethal dose was taken as effective dose (therapeutic dose) for subsequent wound healing activity.
Animals were observed initially after dosing at least once during the first 30 minutes, periodically during the first 24 hours. In all cases death was observed within first 24 hours. Additional observations like changes in skin and for eyes and mucous membranes, and also respiratory, circulatory, autonomic and central nervous systems and rotarod activity and behaviour pattern. Attention was also given to observation of tremors and convulsions.
Preparation of extracts of Anogeissus acuminata
The leaves of Anogeissus acuminate were collected from Tirupati district, Andhra Pradesh, India and authenticated by Dr. K. Madhava Chetty, assistant professor, department of botany, Sri Venkateswara University, Tirupati. The shade dried leaves were cut into small pieces and were powdered mechanically to obtain a coarse powder. The coarse powder was then stored in a clean, dry and airtight container [10]. The powdered material was subjected to maceration. The solvents used were petroleum ether (60°C-80°C), chloroform, methanol and distilled water. The powdered material was evenly packed in a conical flask for extraction for 7 days with different solvents. Then the macerated product was filtered using muslin cloth and resultant product was kept in water bath to get the final product [11]. All extract viz. petroleum ether, methanol, chloroform, and aqueous extract were first subject for the preliminary phytochemical investigation then it was subject to acute toxicity studies and antimicrobial activity. The two extracts, which were showing maximum antimicrobial action, were further subjected to investigate wound healing and antioxidant activities [12].
Results and Discussion
Antioxidant activity
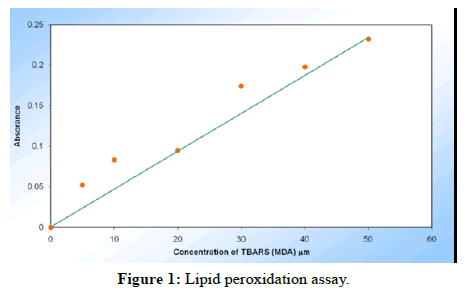
Lipid peroxidation: In 7th and 14th post-wounding day of burn wound model, the TBARS (MDA) levels increased in alcohol extract group (25.07 ± 0.33 μm and 11.22 ± 0.12 μm respectively), whereas decreased in aqueous extract group (18.89 ± 0.23 μm and 8.82 ± 0.24 μm respectively) as compared to control group (Tables 1-3, Figure 1).
| Sample No. | Concentration of MDA (mm) | Absorbance at 535 nm |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 5 | 0.052 |
| 3 | 10 | 0.083 |
| 4 | 20 | 0.095 |
| 5 | 30 | 0.174 |
| 6 | 40 | 0.198 |
| 7 | 50 | 0.232 |
| Sl. No. | Negative | Control | Alcoholic extract | Aqueous extract |
|---|---|---|---|---|
| 1 | 6.7 | 20.5 | 26.2 | 18.3 |
| 2 | 7.4 | 19.4 | 25.4 | 18.6 |
| 3 | 7.8 | 21.1 | 24.7 | 18.9 |
| 4 | 8.3 | 19.9 | 24.5 | 19 |
| 5 | 8.1 | 20.8 | 24.6 | 19.9 |
| 6 | 9.1 | 21.9 | 25.7 | 19.8 |
| Mean ± SEM | 7.90 ± 0.33 | 20.60 ± 0.36 | 25.07 ± 0.33 | 18.89 ± 0.21 |
Note: Values are expressed as mean ± SEM of 6 values; TBARS (MDA) concentration (mm) in burn wound model on 7th post wounding day; ANOVA, F(3/20)=531.1, P<0.0001
| Sl. No. | Negative | Control | Alcoholic extract | Aqueous extract |
|---|---|---|---|---|
| 1 | 6.7 | 9.95 | 11.5 | 8.8 |
| 2 | 7.4 | 9.4 | 10.6 | 9.1 |
| 3 | 7.8 | 9.65 | 11.1 | 7.8 |
| 4 | 8.3 | 9.15 | 10.8 | 9.4 |
| 5 | 8.1 | 9.75 | 11.1 | 8.5 |
| 6 | 9.1 | 9.85 | 10.05 | 9.2 |
| Mean ± SEM | 7.90 ± 0.33 | 9.62 ± 0.12 | 11.01 ± 0.12 | 8.80 ± 0.23 |
Note: Values are expressed as mean ± SEM of 6 values; TBARS (MDA) concentration (mm) in burn wound model on 14th post wounding day; ANOVA, F(3/20)=35.09, P<0.0001
Excision wound study
Wound contraction studies:
I. 0 day results: Alcohol extract shows minimum(P>0.05) wound closure 498.60 ± 0.94 mm2 compared to control group 495.90 ± 1.35 mm2.The mean wound closure of the aqueous extract treated group was 493.89 ± 1.27 mm2 indicating that there was minimum difference (P>0.05) compared to control group 495.90 ± 1.35 mm2 (Table 4).
| Animal No. | Raw wound area | ||
|---|---|---|---|
| Control (normal saline) | Alcoholic extract | Aqueous extract | |
| 1 | 496.92 | 499.95 | 497.93 |
| 2 | 498.94 | 498.94 | 501.97 |
| 3 | 493.88 | 496.92 | 499.95 |
| 4 | 494.9 | 499.95 | 494.9 |
| 5 | 498.94 | 499.95 | 496.92 |
| 6 | 491.87 | 495.91 | 493.89 |
| Mean ± SEM | 495.90 ± 1.35 | 498.60 ± 0.94 | 493.89 ± 1.27 |
| t value | - | 0.1251 | 0.1876 |
| P value | - | P>0.05 | P>0.05 |
Note: Values are expressed as mean ± SEM of 6 values; Raw wound area: ANOVA, F(2/15)=1.419, P>0.05 not significant.
II. 4th day results: Alcohol extract of Anogeissus acuminata leaves showed significant (P<0.05) wound closure 369.50 ± 4.483 mm2 (26.43%) compared to control group 396.45 ± 3.631 mm2 (20.52%).The mean wound closure of aqueous extract treated group was 382.18 ± 2.076 mm2 (23.71%) indicating that there was significant difference (P<0.01) compared to control group 396.44 ± 3.631 mm2 (20.52%) (Table 5).
| Animal No. | Raw wound area | |||||
|---|---|---|---|---|---|---|
| Control (normal saline) | Alcoholic extract | Aqueous extract | ||||
| Raw wound area | % Closure | Raw wound area | % Closure | Raw wound area | % Closure | |
| 1 | 402 | 19.49 | 377.76 | 25.19 | 385.84 | 23.26 |
| 2 | 383.8 | 23.5 | 372.69 | 25.76 | 386.24 | 23.48 |
| 3 | 391.9 | 21.06 | 361.59 | 27.67 | 383.3 | 23.77 |
| 4 | 396.93 | 20.2 | 365.62 | 27.34 | 384.82 | 22.66 |
| 5 | 410.07 | 18.52 | 384.82 | 23.61 | 380.16 | 24.09 |
| 6 | 393.9 | 20.32 | 354.52 | 29 | 372.69 | 24.99 |
| Mean ± SEM | 396.45 ±3.631 | 20.52±0.685 | 369.50 ± 4.483 | 26.43 ±0.785 | 382.18 ±2.076 | 23.71 ±0.319 |
| t value | - | - | 5.327 | 6.574 | 2.82 | 3.549 |
| P value | - | - | P<0.01 | P<0.01 | P<0.01 | P<0.05 |
Note: Values are expressed as mean ± SEM of 6 values; Raw wound area: ANOVA, F(2/15)=14.21, P<0.0003%; Closure: ANOVA, F(2/15)=9.840, P<0.05
III. 8th day results: Alcohol extract of Anogeissus acuminate leaves showed significant (P<0.01) wound closure 153.41 ± 2.20 mm2 (70.15%) compared to the control group 186.04 ± 3.11 mm2 (63.34%).The mean wound closure of the aqueous extract treated group was 163.79 ± 3.08 mm2 (67.99%) indicating that there was significant difference (P<0.05) compared to control group 186.04 ± 3.11 mm2 (63.34%) (Table 6).
| Animal No. | Raw wound area | |||||
|---|---|---|---|---|---|---|
| Control (normal saline) | Alcoholic extract | Aqueous extract | ||||
| Raw wound area | % Closure | Raw wound area | % Closure | Raw wound area | % Closure | |
| 1 | 186.85 | 63.23 | 153.72 | 70.27 | 161.4 | 68.59 |
| 2 | 175.24 | 65.73 | 150.33 | 70.76 | 166.15 | 67.77 |
| 3 | 196.45 | 61.02 | 162.31 | 68.22 | 169.68 | 66.92 |
| 4 | 181.6 | 64.15 | 156.45 | 69.59 | 154.33 | 69.71 |
| 5 | 192.71 | 62.35 | 146.65 | 71.63 | 174.23 | 65.86 |
| 6 | 183.42 | 63.54 | 151 | 70.45 | 156.95 | 69.1 |
| Mean ± SEM | 186.04 ± 3.114 | 63.34 ± 0.64 | 153.41 ± 2.207 | 70.15 ± 0.46 | 163.79 ± 3.084 | 67.99 ± 0.57 |
| t value | - | - | 8.061 | 8.38 | 5.499 | 5.72 |
| P value | - | - | P<0.01 | P<0.01 | P<0.01 | P<0.01 |
Note: Values are expressed as mean ± SEM of 6 values; Raw wound area: ANOVA, F(2/15)=33.93, P<0.0001 ; % Closure: ANOVA, F(2/15)=36.70, P<0.0001
IV. 12th day results: Alcohol extract of Anogeissus acuminate leaves showed significant (P<0.01) wound closure 75.70 ± 2.97 mm2 (82.36%) compared to the control group 102.38 ± 3.24 mm2 (80.66%).The mean wound closure of the aqueous extract treated group was 92.40 ± 1.57 mm2 (85.64%) indicating that there was significant difference (P<0.05) compared to control group 102.38 ± 3.24 mm2 (80.66%) (Table 7).
| Animal No. | Raw wound area | |||||
|---|---|---|---|---|---|---|
| Control (normal saline) | Alcoholic extract | Aqueous extract | ||||
| Raw wound area | % Closure | Raw wound area | % Closure | Raw wound area | % Closure | |
| 1 | 109.59 | 80.12 | 85.85 | 80.91 | 93.93 | 85.55 |
| 2 | 99.38 | 81.32 | 81.31 | 81.81 | 97.47 | 84.23 |
| 3 | 113.12 | 78.76 | 77.57 | 82.52 | 90.86 | 83.73 |
| 4 | 96.46 | 81.89 | 73.23 | 81.88 | 86.36 | 86.83 |
| 5 | 103.83 | 81.04 | 70.6 | 83.87 | 90.9 | 87.64 |
| 6 | 91.91 | 80.84 | 65.65 | 83.18 | 94.94 | 85.87 |
| Mean ± SEM | 102.38 ± 3.24 | 80.66 ± 0.443 | 75.70 ± 2.97 | 82.36 ± 0.428 | 92.4 ± 1.57 | 85.64 ± 0.602 |
| t value | - | - | 6.915 | 2.392 | 2.587 | 7.005 |
| P value | - | - | P<0.01 | P<0.01 | P<0.05 | P<0.01 |
Note: Values are expressed as mean ± SEM of 6 values; Raw wound area: ANOVA, F(2/15)=24.41, P<0.0001; % Closure: ANOVA, F(2/15)=25.76, P<0.0001
V. 16th day results: Alcohol extract of Anogeissus acuminate leaves showed significant (P<0.01) wound closure 38.58±2.01 mm2 (93.39%) compared to the control group 62.25 ± 1.03 mm2 (88.53%).The mean wound closure of the aqueous extract treated group was 46.26 ± 2.07 mm2 (91.82%) indicating that there was significant difference (P<0.01) compared to control group 62.25 ± 1.03 mm2 (88.53%) (Table 8).
| Animal No. | Raw wound area | |||||
|---|---|---|---|---|---|---|
| Control (normal saline) | Alcoholic extract | Aqueous extract | ||||
| Raw wound area | % Closure | Raw wound area | % Closure | Raw wound area | % Closure | |
| 1 | 62.62 | 88.48 | 35.57 | 94.04 | 41.41 | 92.83 |
| 2 | 59.09 | 89.24 | 41.72 | 92.76 | 45.45 | 92.05 |
| 3 | 66.05 | 87.7 | 38.96 | 93.29 | 48.48 | 91.41 |
| 4 | 60.5 | 88.85 | 32.42 | 94.65 | 50.3 | 90.94 |
| 5 | 61.11 | 88.88 | 36.36 | 93.87 | 39.39 | 93.21 |
| 6 | 64.14 | 88.03 | 46.47 | 91.74 | 52.52 | 90.47 |
| Mean ± SEM | 62.25 ± 1.03 | 88.53 ± 0.234 | 38.58 ± 2.01 | 93.39 ± 0.41 | 46.26 ± 2.07 | 91.82 ± 0.43 |
| t value | - | - | 9.35 | 9.1 | 6.31 | 6.15 |
| P value | - | - | P<0.01 | P<0.01 | P<0.01 | P<0.01 |
Note: Values are expressed as mean ± SEM of 6 values; Raw wound area: ANOVA, F(2/15)=45.56, P<0.0001; % Closure: ANOVA, F(2/15)=43.13, P<0.0001
VI. 20th day results: Alcohol extract of Anogeissus acuminate leaves showed significant (P<0.01) wound closure 2.61 ± 0.87 mm2 (99.47%) compared to the control group 21.54 ± 1.07 mm2 (96.82%).The mean wound closure of the aqueous extract treated group was 8.96 ± 1.05 mm2 (98.44%) indicating that there was significant difference (P<0.01) compared to control group 21.54 ± 1.07 mm2 (96.82%) (Table 9).
| Animal No. | Raw wound area | |||||
|---|---|---|---|---|---|---|
| Control (normal saline) | Alcoholic extract | Aqueous extract | ||||
| Raw wound area | % Closure | Raw wound area | % Closure | Raw wound area | % Closure | |
| 1 | 25.45 | 96.03 | 4.06 | 99.88 | 5.45 | 98.48 |
| 2 | 21.67 | 96.83 | 5.26 | 98.96 | 7.27 | 99.59 |
| 3 | 19.9 | 97.13 | 0 | 99.89 | 9.49 | 98.78 |
| 4 | 18.18 | 97.5 | 3.54 | 99.49 | 12.83 | 97.87 |
| 5 | 20.3 | 97.1 | 2.81 | 98.97 | 10.61 | 96.95 |
| 6 | 23.74 | 96.32 | 0 | 99.67 | 8.08 | 98.98 |
| Mean ± SEM | 21.54 ± 1.07 | 96.82 ± 0.22 | 2.6 ± 0.87 | 99.4 ± 0.14 | 8.96 ± 1.05 | 98.44 ± 0.39 |
| t value | - | - | 13.15 | 9.6 | 8.73 | 5.76 |
| P value | - | - | P<0.01 | P<0.01 | P<0.01 | P<0.01 |
Note: Values are expressed as mean ± SEM of 6 values; Raw wound area: ANOVA, F(2/15)=89.56, P<0.0001; % Closure: ANOVA, F(2/15)=46.76, P<0.0001
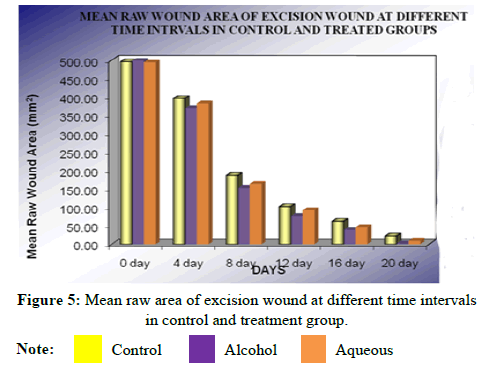
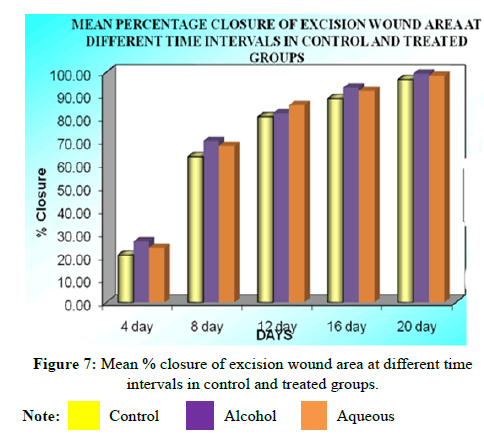
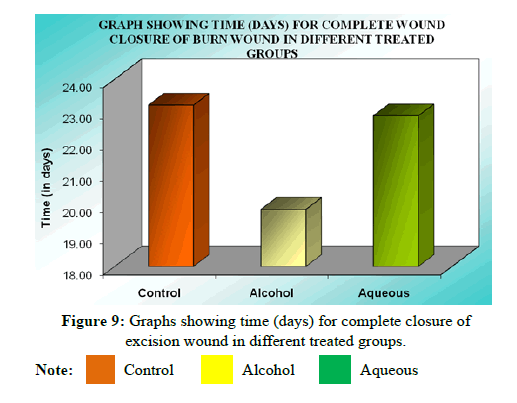
VII. Complete epithelialization (days) results: On further follow up, mean time (days) to complete healing of control was 24.41 ± 0.79 while that of alcohol extract treated group was 19.86 ± 0.66 indicating significant epithelialization (P<0.01) compared to control. Similarly the mean time (days) to complete healing of the wound in aqueous extract treated group was 22.05 ± 0.30, indicating that the difference is similarly significant (P<0.05) compared to the control group (Tables 10 and 11).
| Days | Mean raw wound area (mm2) | Mean percentage closure | ||||
|---|---|---|---|---|---|---|
| Control | Alcoholic extract | Aqueous extract | Control | Alcoholic extract | Aqueous extract | |
| 0 | 495.90 ± 1.35 | 498.60 ± 0.94 | 493.89 ± 1.27 | - | - | - |
| 4 | 396.45 ± 3.63 | 369.50 ± 4.48 | 382.18 ± 2.07 | 20.52 ± 0.68 | 26.43 ± 0.78 | 23.71 ± 0.31 |
| 8 | 186.04 ± 3.11 | 153.41 ± 2.20 | 163.79 ± 3.08 | 63.34 ± 0.64 | 70.15 ± 0.46 | 67.99 ± 0.57 |
| 12 | 102.38 ± 3.24 | 75.70 ± 2.97 | 92.40 ± 1.57 | 80.66 ± 0.44 | 82.36 ± 0.42 | 85.64 ± 0.60 |
| 16 | 62.25 ± 1.03 | 38.58 ± 2.01 | 46.26 ± 2.07 | 88.53 ± 0.23 | 93.3 ± 0.41 | 91.82 ± 0.43 |
| 20 | 21.54 ± 1.07 | 2.61 ± 0.87 | 8.96 ± 1.05 | 96.82 ± 0.22 | 99.47 ± 0.14 | 98.44 ± 0.39 |
Note: Values are expressed as mean ± SEM of 6 values; *P<0.01, **P<0.05 compared to control; ANOVA followed by Dunnet’s‘t’ test.
| Animal No. | Control | Alcoholic extract | Aqueous extract |
|---|---|---|---|
| 1 | 27.27 | 19.19 | 22.22 |
| 2 | 23.23 | 20.2 | 23.23 |
| 3 | 24.24 | 18.18 | 21.21 |
| 4 | 23.23 | 21.21 | 22.22 |
| 5 | 22.22 | 18.18 | 21.21 |
| 6 | 26.26 | 22.22 | 22.22 |
| Mean ± SEM | 24.41 ± 0.79 | 19.86 ± 0.30 | 22.05 ± 0.66 |
| t-value | - | 5.103 | 2.646 |
| P-value | - | P<0.01 | P<0.05 |
Note: Values are expressed as mean ± SEM of 6 values; Time for complete wound closure: ANOVA, F(2/15)=13.02, P<0.005
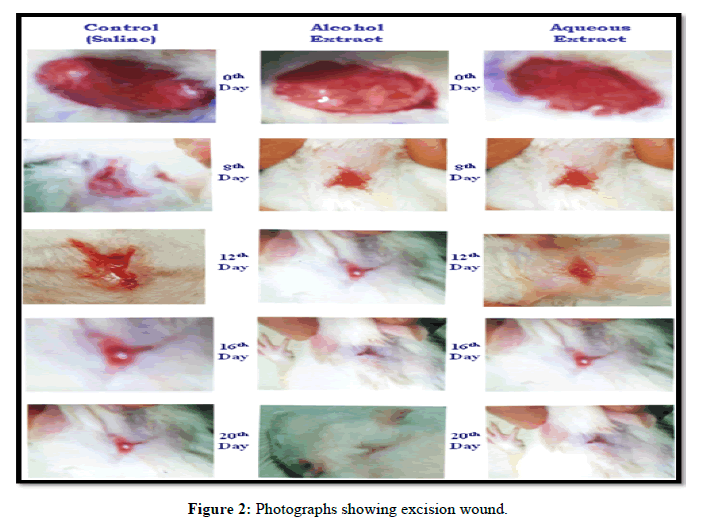
Statistical analysis of the result obtained at 20th post wounding day by ANOVA followed by Dunnet’s “t” test showed that there was significant difference between all the groups,<0.0001. Both the extracts showed significant wound contraction and the alcohol extract was found to be more effective than aqueous extract (Figure 2).
Incision wound study
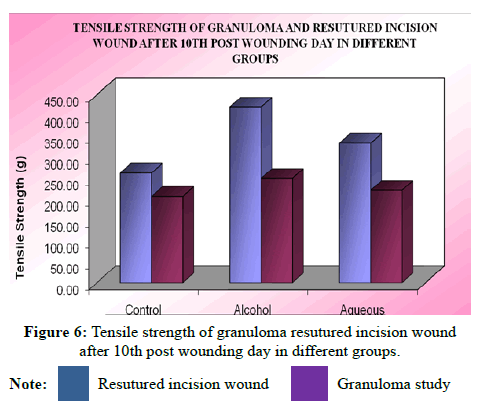
The breaking strength of 10-day-old re-sutured incision wound in control and various treated groups were studied. The mean breaking tensile strength of wounds in control animal was 420.7 ± 10.36 g whereas aqueous extract treated group was 263.0 ± 7.88 g indicating a significant increase (P<0.01) in breaking tensile strength of incision wound compared to control group, while in alcohol extract treated group it was 334.4 ± 9.10 g indicating a more significant increase (P<0.01) in breaking strength of incision wound compared to control group 420.7 ± 10.36 g [13].
Statistical analysis of the results by ANOVA followed by Dunnet’s t test showed that there was significant difference between all the groups (F=52.50), P<0.0001 and the alcohol extract was found to be highly effective than the aqueous extract.
Ganuloma studies
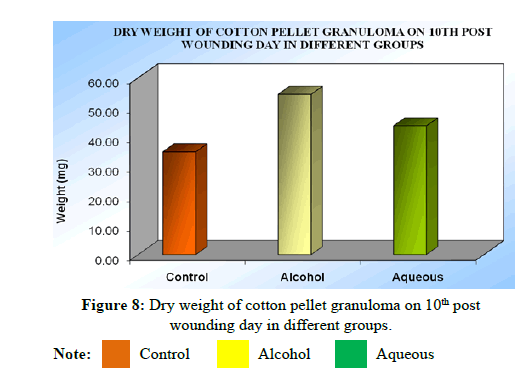
Dry weight (mg) of cotton pellet granuloma: The dry weight of cotton pellet was expressed as mg of body weight of corresponding animal on 10th day of wounding. Mean dry weight of cotton pellet granuloma in control group was 35.90 ± 1.18 mg, whereas, it was 53.38 ± 3.28 mg in alcohol extract treated group which showed a significant (P<0.01) increase in granuloma weight in treated group compared control group. In case of aqueous extract treated group, it was 44.68 ± 2.63 mg, indicating that there was no significant increase (P>0.05) of granuloma formation compared to control group 35.90 ± 1.18 mg (Table 12).
| Animal No. | Control | Alcoholic extract | Aqueous extract |
|---|---|---|---|
| 1 | 36.56 | 60.36 | 54.84 |
| 2 | 40.44 | 56.46 | 38.78 |
| 3 | 39.96 | 41.61 | 36.87 |
| 4 | 33.13 | 42.68 | 47.17 |
| 5 | 31.82 | 54.74 | 43.83 |
| 6 | 33.53 | 64.44 | 46.56 |
| Mean ± SEM | 35.90 ± 1.18 | 53.38 ± 3.28 | 44.68 ± 2.61 |
| t-value | - | 5.385 | 2.425 |
| P-value | - | P<0.01 | P>0.05 |
Note: Values are expressed as mean ± SEM of 6 values; Dry weight of cotton pellet granuloma: ANOVA, F(2/15)=14.55, P<0.0003
Tensile strength study on granuloma pith: The mean breaking strength of granuloma pith in control animal was 206.90 ± 8.36 g and alcohol extract treated group was 249.90 ± 9.15 g indicating that there was significant (P<0.05) increase in breaking strength of granuloma pith compared to control group. The mean breaking tensile strength of granuloma pith in aqueous extract treated group 263.0 ± 7.38 g was not significantly (P>0.05) increased compared to control group 206.90 ± 8.36 g (Table 13).
| Animal No. | Granuloma study | Re-sutured incision wound | ||||
|---|---|---|---|---|---|---|
| Control | Alcoholic extract | Aqueous extract | Control | Alcoholic extract | Aqueous extract | |
| 1 | 194.5 | 278.8 | 249.8 | 241.2 | 389.7 | 298.9 |
| 2 | 214.8 | 245.9 | 194.2 | 264.5 | 445.5 | 321.8 |
| 3 | 189.9 | 230.4 | 253.2 | 254.2 | 412.2 | 380.3 |
| 4 | 224.9 | 265.5 | 198.5 | 249.8 | 395.6 | 294.9 |
| 5 | 183.5 | 219.2 | 201.9 | 286.9 | 449.4 | 338.8 |
| 6 | 233.8 | 259.7 | 231.1 | 281.3 | 431.7 | 371.5 |
| Mean ± SEM | 206.9 ± 8.36 | 249.9 ± 9.15 | 221.5 ± 10.89 | 263.0 ± 7.88 | 420.7 ± 10.36 | 334.4 ± 9.10 |
| t-value | - | 3.192 | 1.08 | - | 9.934 | 4.497 |
| P-value | - | P<0.05 | P>0.05 | - | P<0.01 | P<0.01 |
Note: Values are expressed as mean ± SEM of 6 values;Tensile strength of resutured incision wound: ANOVA, F(2/15)=49.49, P<0.0001;Tensile strength of granuloma: ANOVA, F(2/15)=5.27, P<0.01.s
Statistical analysis of the results by ANOVA followed by Dunnet’s ‘t’ test showed that there was significant increase in tensile strength of aqueous and aqueous extract treated group as compared to control (F=5.28). Alcohol extract treated group was found to be highly effective than aqueous extract (Figures 3 and 4).
Hydroxyproline estimation
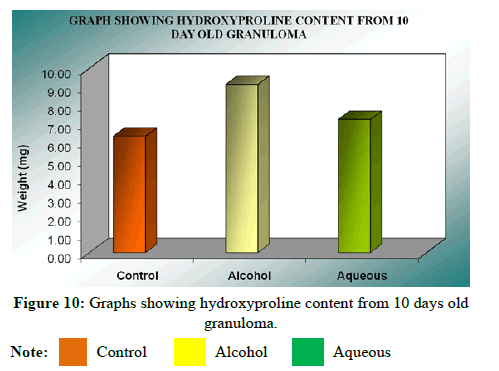
Hydroxyproline content was expressed as microgram (μg) per 300 mg of wet granulation tissue. The mean hydroxyproline content in control was 9.14 ± 0.20 μg, while it was 7.22 ± 0.21 μg in alcohol extract treated group indicating that there was a significant (P<0.01) increase in hydroxyproline content compared to control group. However, In case of aqueous extract treated group, it was 6.33 ± 0.10 μg, indicating there is less significant increase than alcohol extract compared to control. Statistical analysis of the results by ANOVA followed by Dunnet’s‘t’ test showed that there was significant difference between all the groups (F=58.29), P<0.001 and alcohol extract was found to be highly effective (Table 14).
| Animal No. | Control (mg) | Alcoholic extract | Aqueous extract |
|---|---|---|---|
| 1 | 9.85 | 7.24 | 6.53 |
| 2 | 8.67 | 6.53 | 6.63 |
| 3 | 9.28 | 7.04 | 6.08 |
| 4 | 8.47 | 6.83 | 6.02 |
| 5 | 8.98 | 7.75 | 6.43 |
| 6 | 9.59 | 7.9 | 6.32 |
| Mean ± SEM | 9.14 ± 0.20 | 7.22 ± 0.21 | 6.33 ± 0.10 |
| t-value | - | 8.57 | 5.39 |
| P-value | - | P<0.01 | P<0.01 |
Note: Values are expressed as mean ± SEM of 6 values; Hydroxyproline content of granuloma: ANOVA, F(2/15)=58.29, P<0.0001
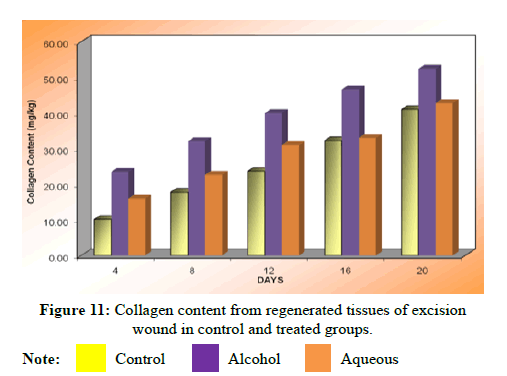
Collagen content
The collagen content was estimated from regenerated tissues for control as well as treated groups. There was a significant increase (P<0.01) in collagen content on 4th, 8th, 12th, 16th and 20th days in alcohol extract treated group compared to the control group. The increase in collagen content in aqueous extract treated group was also significant (P<0.01) as compared to the control group. Statistical analysis of the results by ANOVA followed by Dunnet’s ‘t’ test showed that there was significant difference between control and treated group P<0.0001 and alcohol extract was found to be highly effective as compared to the aqueous extract (Table 15).
| Days | Control (normal saline) | Alcoholic extract | Aqueous extract |
|---|---|---|---|
| 4 | 10.11 ± 0.82 | 23.43 ± 1.682** | 15.96 ± 0.6540** |
| 8 | 17.79 ± 0.97 | 32.09 ± 0.9609** | 22.64 ± 0.8141** |
| 12 | 23.63 ± 1.32 | 39.96 ± 06658** | 31.02 ± 0.6652** |
| 16 | 32.23 ± 0.87 | 46.59 ± 0.9440** | 32.98 ± 0.3569** |
| 20 | 40.97 ± 0.70 | 52.41 ± 0.6470** | 42.73 ± 0.7554 |
Note: Values are expressed as mean ± SEM of 6 values; Collagen content: ANOVA, P<0.0001;**P<0.01
Histopathological results
Histopathological examinations were carried out in the regenerated tissues (collected from the wounds of both the control and test groups) on the various post-wounding day [14].
Epithelialization: From the result, it was observed that there was difference in epithelialization on the 12th post-wounding day between control and treated groups. The alcohol extract treated groups showed significant (P<0.01) increase in epithelialization compared to control group (Tables 16 and 17).
| Days | Epithelialization | Collagenisation | Inflammation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Alcoholic extract | Aqueous extract | Control | Alcoholic extract | Aqueous extract | Control | Alcoholic extract | Aqueous extract | |
| 4th | 0.46 ± 0.13 | 0.92 ± 0.22 | 0.75 ± 0.22 | 0.38 ± 0.13 | 0.48 ± 0.03 | 0.46 ± 0.05 | 1.75 ± 0.27 | 2.25 ± 0.27 | 2.32 ± 0.18 |
| 8th | 0.92 ± 0.10 | 2.33 ± 0.26 | 1.67 ± 0.26 | 0.50 ± 0.00 | 1.75 ± 0.22 | 1.65 ± 0.38 | 1.83 ± 0.26 | 2.42 ± 0.38 | 2.38 ± 0.42 |
| 12th | 4.83 ± 0.27 | 4.925 ± 0.27** | 4.75 ± 0.27* | 1.75 ± 0.27 | 2.50 ± 0.32** | 2.25 ± 0.42* | 1.92 ± 0.38 | 1.75 ± 0.27 | 1.82 ± 0.32 |
| 16th | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 2.58 ± 0.38 | 3.08 ± 0.20 | 2.98 ± 0.31 | 0.83 ± 0.20 | 0.71 ± 0.19 | 0.81 ± 0.24 |
| 20th | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 2.92 ± 0.42 | 4.08 ± 0.20 | 3.67 ± 0.26 | 0.71 ± 0.10 | 0.42 ± 0.13 | 0.58 ± 0.13 |
Note: Values are mean (±) SEM of 6 readings; ANOVA followed by Dunnet’s‘t’ test: *P<0.01 and ** P<0.05 when compared to the control.
| Days | Neo-vascularity | Cellularity | ||||
|---|---|---|---|---|---|---|
| Control | Alcoholic extract | Aqueous extract | Control | Alcoholic extract | Aqueous extract | |
| 4th | 1.17 ± 0.26 | 1.91 ± 0.46 | 1.83 ± 0.41 | 1.78 ± 0.29 | 2.48 ± 0.39 | 2.42 ± 0.38 |
| 8th | 3.33 ± 0.26 | 2.54 ± 0.3** | 2.50 ± 0.32 | 1.83 ± 0.26 | 2.62 ± 0.36** | 2.46 ± 0.32 |
| 12th | 2.42 ± 0.38 | 2.12 ± 0.22 | 2.08 ± 0.20 | 3.08 ± 0.20 | 3.67 ± 0.27* | 2.55 ± 0.26 |
| 16th | 1.83 ± 0.26 | 1.02 ± 0.19 | 1.08 ± 0.20 | 3.54 ± 0.25 | 3.58 ± 0.42 | 3.42 ± 0.38 |
| 20th | 0.58 ± 0.13 | 0.58 ± 0.13 | 0.42 ± 0.13 | 3.67 ± 0.26 | 3.51 ± 0.32 | 3.53 ± 0.32 |
Note: Values are mean (±) SEM of 6 readings; ANOVA followed by Dunnet’s‘t’ test: *P<0.01 and ** P<0.05 when compared to the control.
Inflammation: From the results obtained there was considerable difference in the inflammatory response between the test and control group except for the significant (P<0.01) decrease in alcohol extract treated group. Other groups did not show any significance.
Collagen content: Collagen content was significantly (P<0.01) increased in alcohol extract treated group on 12th day and there was significantly (P<0.05) increase in collagen content in aqueous extract treated group.
Neovascularization: Alcohol extract treated group showed significant (P<0.01) increase in neovascularization compared to control. Aqueous extract treated group was not significant (P>0.05) increase in neovascularization [15].
Cellularity (fibroblast): Alcohol extract treated group showed significant (P<0.01) change in the fibroblast content compared to control on 12th post wounding day. In aqueous extract treated group did not show any significant change in fibroblast content on 12th post wounding day (Figures 5-11).
Conclusion
The relationship between man and plants has been close throughout the development of human culture with the disease in the understanding of human disease there has been continued interest in the drugs from the plant kingdom.
The wound healing studies of alcoholic and aqueous extracts showed significant wound healing activity at 300 mg/kg dose as compared to control. From the dead space wound studies, it was found that alcoholic and aqueous extracts of Anogeissus acuminata significantly increased the tensile strength of granuloma and there was significant increase in the weight of cotton pellet granuloma than the control.
For wound healing studies, it is clearly understood by histopathological studies that there was significant difference between the test and control on all observed days. The inflammatory response indicates the entire process of inflammation results in the stimulation of fibroblast for synthesis of collagen. The cellularity was not different quantitatively in the test and control group of aqueous extract treated groups, but it showed a quantitative difference in collagen content. So in test wounds, the fibroblast content resulted in faster synthesis of collagen compared to control, which is parallel with maximum rate of wound contraction.
Reports suggest that restoration of tissue continuity after injury and strengthening of repairing tissue depends primarily on the function of the fibroblast. The cell probably comes from the tissues immediately surrounding the wound area but may also originate from other local or distant mesechymal cells. Moreover, fibroblast can migrate during the healing process and become contractile as myofibroblast. This contractile ability contributes to healing of wounds. It is known that collagen is the major product of the fibroblast.
The present investigations were carried out to evaluate anti-oxidant and the wound healing activity of Anogeissus acuminate. These extracts are also significantly active in different wound healing models. This is further confirmed by the histochemical (hydroxyproline, collagen) and histopathological studies.
The drug also has a significant antioxidant activity, which probably may be through blocking the production of ROS generation from the body lipids. The wound healing activities are may be due to the presence of active principles like tannins, alkaloids, flavonoids and saponins. However, further studies are needed to confirm the constituents responsible for the activity.
References
- Modi A. Eastern. Pharm. 1984; 24(315): 47.
- Tandon V, Kappor B, Gupta BM. Indian. J. pharmacol. 2004; 36 (2): 99-100.
- http://en.wikipedia.org/wiki/Antioxidant. 2016.
- Yeo JH, Lee KW, Kim HC, Lyum Y, Kim A, Kim SY. Biol. Pharm. Bull. 2000; 23: 1220-1223.
- Walter I. Gen. Pathol. 5(7): 165-180.
- Kakali S, Pulok K. J. Ethnopharmacol. 1997; 56: 139-144.
- Verma PK, Pankaj NK. Vet. J. 2008; 3(1).
- OECD. Tech. Chem. 2000.
- Diener W, Mischke U, Kayser D, Schlede E. Arch. Toxicol. 1995;69: 729-734.
- Chrungoo VJ, Singh K, Singh J. Ind. J. Exp. Biol. 1997; 35: 603-610.
- Curtis D, Klassen A. J. Pharm. 2001: 1877-1802.
- Kritikar KR, Basu BD. J. Med. Pharm. 1980: 589-591.
- Kokate CK, Purohit AP, Gokhale SB. J. Pharma. 1996.
- Chattarjee A. J. Pharm. 2010; 3: 163-165.
- The Wealth of India. CSIR. 1950; 9: 111-120.
Manuscript Submission
Submit your manuscript at Online Submission System
Google scholar citation report
Citations : 1101
International Journal of Pharmacy received 1101 citations as per google scholar report
International Journal of Pharmacy peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- HINARI
- Index Copernicus
- Google Scholar
- The Global Impact Factor (GIF)
- Polish Scholarly Bibliography (PBN)
- Cosmos IF
- Open Academic Journals Index (OAJI)
- Directory of Research Journal Indexing (DRJI)
- EBSCO A-Z
- OCLC- WorldCat
- MIAR
- International committee of medical journals editors (ICMJE)
- Scientific Indexing Services (SIS)
- Scientific Journal Impact Factor (SJIF)
- Euro Pub
- Eurasian Scientific Journal Index
- Root indexing
- International Institute of Organized Research
- InfoBase Index
- International Innovative Journal Impact Factor
- J-Gate