HTML
Short Communication - (2022) Volume 12, Issue 2
Dose the COVID-19 Coronavirus will be Protected by the Bordetella pertussis Vaccine?
Camila Espejo**Correspondence: Camila Espejo, Department of Pharmacy and Research, Utah Valley University, Orem Utah, United States, Email:
Received: 07-Feb-2022, Manuscript No. IJP-22-60683; Editor assigned: 11-Feb-2022, Pre QC No. IJP-22- 60683(PQ); Reviewed: 21-Feb-2022, QC No. IJP-22-60683; Revised: 28-Feb-2022, Manuscript No. IJP-22- 60683(R); Published: 07-Mar-2022, DOI: 10.37532/2249-1848-22.12.08
About the Study
According to the World Health Organization (WHO), the new Coronavirus Disease (COVID-19) has caused 21,65,500 confirmed cases and 145705 deaths in over 185 countries as of today. Despite the tremendous efforts from several top health institutes throughout the world to develop a vaccine, WHO Director-General Tedros Adhanom Ghebreyesus said this could take 18 months before a coronavirus vaccine is readily available. Because no single institution has the capacity or facilities to develop, test, and manufacture a vaccine on its own, the limits extend beyond vaccine development to where phase 3 trials will be conducted and who will manufacture the vaccine at scale.
As a result, identifying existing, approved therapies or a protective approach with proven safety profiles would be an ideal solution to address the immediate need to reduce rising mortality. Thousands of lives will be lost before a vaccine can be developed and tested, given the rapid spread of COVID-19. As a result, an alternative solution to this crisis must be found. We propose putting the Bordetella pertussis vaccine to the test to see if it can protect against COVID- 19. The following is a summary of our observational theory:
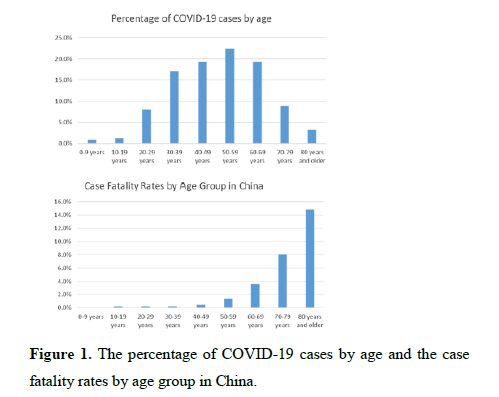
1. The percentage of COVID-19-infected patients age 0 to 19 years old in a sample of COVID-19-infected patients in China was reported to be around 1% [1].
2. The case fatality rates for the age groups of 0-9 years and 10-19 years has also been reported to be 0 percent and 0.2 percent, respectively (Figure 1) [1].
3. In a recent multicentre study, age, the presence of underlying diseases, the presence of secondary infection, and elevated inflammatory indicators in the blood all were found to be predictors of a fatal outcome in COVID-19 cases, with interleukin-6 (IL-6) being significantly higher in non-survivors vs. survivors (P<0.0001). [2]
4. BPZE1 one of the main strains attenuated or detoxified in the attenuated virulent B. pertussis vaccine has previously been shown to protect against viral infection by altering the viral response and increasing natural mucosal resistance [3,4]. Patients infected with the Respiratory Syncytial Virus (RSV), BPZE1 has an useful immunomodulatory impact, providing polyvalent protection through both specific and nonspecific immunologic effects. This beneficial effect can last until adulthood after priming with BPZE1 during the neonatal era.
5. The B. pertussis BPZE1 strain has been found to have antiinflammatory capabilities, making it an excellent candidate for use as a new highly efficient vaccine. It is recommended that a long-acting preventive agent against severe and deadly pneumonitis induced by H3N2 and H1N1 influenza A viruses [5] be used. Exaggerated cytokine-mediated inflammation of three proinflammatory cytokines and chemokines, namely IL-1, IL-6, and granulocyte-macrophage colony-stimulating factor (GMCSF), was decreased in BPZE1-treated rats, resulting in protection against influenza virus-induced severe pneumonitis [5].
6. Pertussis vaccination is recommended at 2, 4, and 6 months in the United States in 2019, according to the National Center for Immunization and Respiratory Disease, with another dose at 15-18 months, a booster dose at 4-6 years, and a final booster dose at 11-12 years.
7. The pertussis vaccine provides good protection for the first 2 years after vaccination, but beyond that, protective immunity begins to diminish. It is followed by a booster of tetanus and diphtheria only (Td) every 10 years after the last dose at 11-12 years.
8. COVID-19 resembles Severe Acute Respiratory Syndrome in certain ways (SARS). SARS had a pattern among children that was comparable to COVID-19, with only a few confirmed cases and no child deaths reported. Scientists are still unclear why this occurred, however a similar concept may be proposed.
9. In 2011, the Centers for Disease Control and Prevention (CDC) recommended pertussis, tetanus, and diphtheria toxoid vaccination boosters during each pregnancy.This may explain the gender differences in COVID-19 incidence, severity, and death in patients [6].
Finally, we can hypothesize that the B. pertussis vaccine's protective effect, which dampens cytokine storms, is responsible for the low fatality of COVID-19 patients in the population under 19 years old. The lower prevalence in the younger population may be due to a benign presentation that does not require hospitalisation. Moreover, B. pertussis that has been attenuated can be used as a mucosal vaccine delivery system. The exponentially increased incidence of COVID-19 in older ages when the pertussis vaccine effect begins to fade, which takes 4-7 years after the last booster dose of pertussis vaccine, backs up this theory. This theory gives hope for avoiding future cases of COVID-19, and it must be supported by research studies to demonstrate the benefits of administering a booster dose of B. pertussis vaccine along with diphtheria and tetanus toxoid to protect against COVID-19 infection or, or in the very least, reduce the severity of infection and thus improve the outcome, especially in the elderly or high-risk population. We offer this theory to the scientific community in order to raise awareness of it and to provide us with realistic and experimental evidence to support that claim.
References
- Max R, Ritchie H, Ortiz-Ospina E, et al. Our World in data. 2020.
- Ruan Q, Yang K, Wang W, et al. Int Care Med. 2020;46:846-848.
[CrossRef] [Google Scholar] [Pubmed]
- Fischer JE, Johnson TR, Peebles RS. J Infect Dis. 1999;180:714-719.
[CrossRef] [Google Scholar] [Pubmed]
- Schnoeller C, Roux X, Sawant D, et al. Am J Respir Crit Care Med. 2014;189:194-202.
[CrossRef] [Google Scholar] [Pubmed]
- Li R, Lim A, Phoon MC, et al. J Virol. 2010;84:7105-113.
[CrossRef] [Google Scholar] [Pubmed]
- Kline JM, Lewis WD, Smith EA, Tracy LR, Am Fam Phy. 2013;88:507-514.
[Google Scholar] [Pubmed]
Manuscript Submission
Submit your manuscript at Online Submission System
Google scholar citation report
Citations : 1101
International Journal of Pharmacy received 1101 citations as per google scholar report
International Journal of Pharmacy peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- HINARI
- Index Copernicus
- Google Scholar
- The Global Impact Factor (GIF)
- Polish Scholarly Bibliography (PBN)
- Cosmos IF
- Open Academic Journals Index (OAJI)
- Directory of Research Journal Indexing (DRJI)
- EBSCO A-Z
- OCLC- WorldCat
- MIAR
- International committee of medical journals editors (ICMJE)
- Scientific Indexing Services (SIS)
- Scientific Journal Impact Factor (SJIF)
- Euro Pub
- Eurasian Scientific Journal Index
- Root indexing
- International Institute of Organized Research
- InfoBase Index
- International Innovative Journal Impact Factor
- J-Gate